Hepatoprotective Potential of Herbal Plants and Identifying Important Representatives of Phytoconstituents used for the Treatment of Hepatic Diseases: A Review
Volume 1, Issue 1, Pages 29-38
Abstract
The liver is a vital organ of paramount importance involved in maintaining metabolic functions and detoxifying the exogenous and endogenous challenges like xenobiotics, drugs, viral infections and chronic alcoholism. Despite the continuous and rapid advances in modern medicine, liver dysfunction is still a worldwide health problem. Thus, exploring more therapeutic alternatives without severe undesirable side effects is necessary, and medicinal plants should be utilized and reevaluated because of their abundant resources. There has been increasing interest in the exploration on flavonoids from plant sources because of their multipurpose health benefits stated in numerous epidemiological studies. Subsequently flavonoids are directly associated with human dietary components and health, there is a need to evaluate the structure and function relationship. Flavonoids aid in combating oxidative stress and act as growth regulators. For pharmaceutical purposes, cost effective bulk production of different types of flavonoids has been made promising with the help of microbial biotechnology. This review highlights the hepatoprotective potential of flavonoids and their beneficial roles in human health.
Author
Corresponding Author
Gaurav Dubey
History
Received 04 November 2025
Revised 20 November 2025
Accepted 29 November 2025
Keywords
Flavonoids, Hepatoprotective activity, Liver, Alcohol, Hepatitis, Hepatic diseases.
Open Access
This is an open access article under the CC BY license https://creativecommons.org/licenses/by/4.0/.
INTRODUCTION
Liver disease is a severe problem in developing countries and a cause of morbidity and mortality throughout the world. Liver ailments are usually caused by hepatitis A, B, C viruses, carbon tetrachloride (CCl4), high doses of paracetamol, thioacetamide (TAA) and specific chemotherapeutic agents, etc. [1]. It has been estimated in recent reports that 10% of the world population is affected with liver diseases including hepatitis, alcoholic steatosis, fibrosis, liver cirrhosis and hepatocellular carcinoma. Morbidity and mortality from liver diseases are major public health problems worldwide [2]. It also undergoes profound pathological changes, such as diabetes or obesity [3]. Liver involvement is generally characterized by increased specific bio-chemical parameters, such as transaminases, and a decrease in antioxidant enzymes [4]. Several etiological factors, such as alcohol, hepatitis B or C virus infections, or exposure to aflatoxin-B1, can lead to chronic inflammation and tissue damage, leading to tissue necrosis.
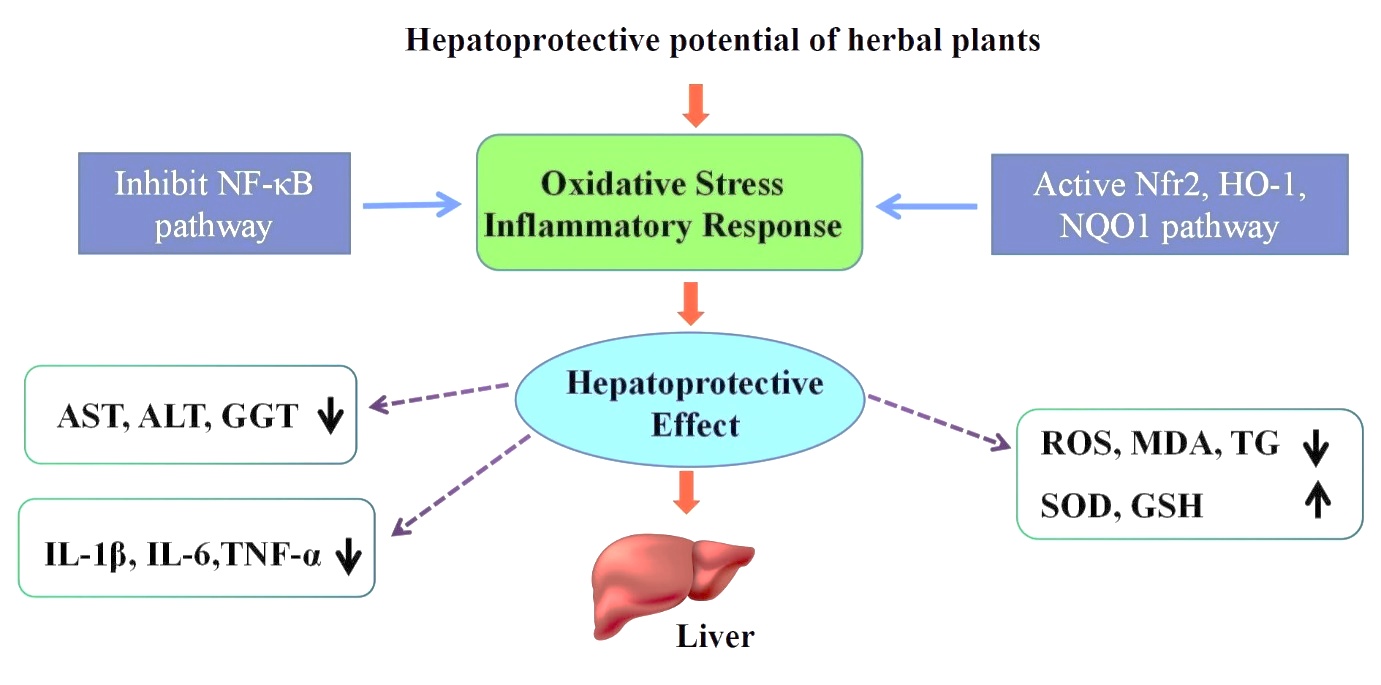
Nowadays, modern medicine offers alternatives for treating these pathologies, but despite the advances, few effective drugs that protect and regenerate hepatic cells exist. Moreover, existing treatments can cause adverse effects, making the therapy of these pathologies even harder. Thereby, the need to identify novel alternatives for treating hepatic diseases and protecting the liver appears necessary to develop novel agents with high efficiency and superior safety profile. Furthermore, mechanisms that underlie the hepatoprotective activity are strongly related to the capacity of antioxidants to scavenge reactive oxygen species (ROS) produced by the metabolic conversion of xenobiotics and induce oxidative stress and damage to the liver tissue (Figure 1).
India is the land of medicinal plants and it is essential to register the data by scientific research on such plants that could be of clinical importance. Treating various health issues like cancer, arthritis, and diabetes has been documented in the Ayurvedic medical system practiced mainly in the Asian continents for more than 5000 years. Indian traditional medication like Ayurvedic, Siddha and Unani are predominantly based on plant materials. Herbal drugs have gained importance and popularity recently because of their safety, efficacy and cost effectiveness. However, modern drugs have very little to offer for alleviation of hepatic ailments, whereas most important representatives of phyto-constituents used for liver diseases chiefly on a regional basis include drugs like silymarine (Silybum marianum) and catechin (Anacardium occidentalis) in Europe, Glycyrrhizin (Glycyrrhiza glarbra) in Japan and chizandrins (Schizandra chinesis) in China. Medicinal plant extracts exhibit protective mechanism against oxidative stress by enhancing antioxidant enzyme activities and averting GSH depletion [5]. Flavonoids as secondary metabolites are widely available in the herbal extracts, which are found to be responsible for antioxidant functions [6]. Enriched foods with flavonoids, natural treatments from food or medicinal plants are considered effective and safe for hepatotoxicity [7]. Numerous flavonoids such as catechin, apigenin, quercetin, naringenin, rutin, and venorutonare reported for their hepatoprotective activities [8]. There has been growing interest in the research on flavonoids from plant sources because of their versatile health benefits reported in various epidemiological studies. Subsequently flavonoids are directly associated with human dietary ingredients and health, there is a need to evaluate the structure and function relationship. The bioavailability, metabolism and biological activity of flavonoids depend upon the configuration, total number of hydroxyl groups and substitution of functional groups about their nuclear structure. Fruits and vegetables are the primary dietary sources of flavonoids for humans, along with tea and wine. Most recent research has focused on the health aspects of flavonoids for humans. Many flavonoids are shown to have anti-oxidative activity, free radical scavenging capacity, coronary heart disease prevention, hepatoprotective, anti-inflammatory and anticancer activities, while some flavonoids exhibit potential antiviral activities.
The current review study explores potential flavonoids with hepatoprotective activity and the data regarding the nature of phytochemicals and their mechanism of action during in-vivo/in-vitro studies for their hepatoprotective effectiveness.
METHODOLOGY
Literature search was conducted from the published data, using Elsevier-Science direct, SpringerLink, Wiley Interscience (Wiley), Pubmed and Google Scholar. The search included the following keywords: medicinal plants, herbal plants, cross-referenced with the keywords: hepatoprotective, liver diseases, hepatic diseases and hepatoprotective activity.
HEPATOPROTECTION
Liver diseases have become one of the significant causes of morbidity and mortality all over the world. However, we do not have satisfactory liver protective drugs in allopathic medical practice for severe liver disorders. Numerous plants and traditional formulations are available to treat liver diseases. About 600 commercial herbal formulations with claimed hepato-protective activity are being soldworldwide. Liver damage is always associated with cellular necrosis, an increase in tissue lipid peroxidation and depletion in the tissue GSH levels. In addition, serum levels of many biochemical markers like ALT, AST, triglycerides, cholesterol, bilirubin and alkaline phosphatase are elevated [9]. It has been projected that some 200 million chronic carriers of the hepatitis B virus, of which 40% are expected eventually due to hepatocellular carcinoma and 15% of cirrhosis. In recent years in vivo and in vitro test models have been developed to evaluate plants for their anti-hepatotoxic activities [10]. The testing may be carried out by causing liver damage in experimental animals and estimating the beneficial effects of treatment as measured by liver function test or by removing part of the liver and measuring the regeneration rate. In such liver damage, the serum level of the liver enzymes, particularly serum glutamic-oxaloacetic transaminase and serum glutamic-pyruvic transaminase is raised and extended of its control by the anti-hepatotoxic drug under test is used as a basis for estimation [10]. It has been reported that about 170 phytoconstituents isolated from 110 plants belonging to 55 families were stated to possess liver protective activity.
Conventional medicine does not provide many remedies for hepatitis, cirrhosis, liver damage by toxins or biliary tract disorders [11]. There are no definite allopathic medicines used as hepatoprotective, although different research works are going on some drug like that Rimonabant chemically described as N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide, is selective endocannabinoid (CB1) receptor antagonist, inhibits the pharmacological effects of cannabinoids agonists in vitro and in vivo and has hepato-protective activity against hepatotoxicant like ethanol. It has seen that administration of rimonabant at 2.5 mg/kg, 5 mg/kg and 10 mg/kg dose levels attenuated the increased level of the serum enzymes produced by ethanol and caused a subsequent recovery towards normalization almost like that of Silymarin treatment [12]. Steroids like corticosteroids are under the study for their hepatoprotective action. Many other therapeutic interventions have been studied in alcoholic hepatitis but have not been able to show convincing benefits, including trials of anti-oxidants (vitamin E and combination antioxidants), anti-fibrotics (colchicine), anti-thyroid drugs, promoters of hepatic regeneration (insulin and glucagon), anabolic steroids (oxandrolone and testosterone), as well as calcium channel blockers (amlodipine), polyunsaturated lecithin, and many complementary and alternative medicines [13]. A number of other agents have been verified in patients including propylthiouracil to decrease the hyper-metabolic state induced by alcohol [14].
The usage of natural remedies for the treatment of the liver diseases has a long history and medicinal plants and their derivatives are still used all over the world in one form or the other for this purpose. Medicinal plants are more widely used than allopathic drugs as hepatoprotectives because of them are inexpensive, better cultural acceptability, better compatibility, with the human body and minimal side effects. Medicinal plants possessed hepatoprotective activity through many mechanisms, included antioxidant properties of medicinal plants, enhancement of antioxidant defense (superoxide dismutase, catalase and glutathione peroxidase activity) [15,16], reduced peroxidation [17,18], reversed hepatic fibrosis via enhancement of the expression of matrix metalloproteinase and removal of collagen deposits, with attenuation of hepatic stellate cells activation [19], their anti-inflammatory activity and attenuation of many inflammatory processes, antifibrotic properties of plants and stimulation of extracellular matrix degradation [15].
Hepatoprotective Plants
In recent years, many researchers have examined the effects of plants used traditionally by many folklore remedies from plant origin have long been used for the treatment of liver diseases indigenous healers and herbalists support liver function and treat conditions of the liver. In most cases, research has confirmed traditional experience and wisdom by discovering the mechanisms and mode of action of these plants and reaffirming the therapeutic effectiveness of certain plants or plant extracts in clinical studies. Several hundred plants have been examined for use in various liver disorders (Table 1).
| Name of plant | Plant extract | Phytoconstituents | Experimental models | Study outcome | Refs. | ||
|---|---|---|---|---|---|---|---|
| Allium cepa | Aqueous extract of fresh bulbs | Flavonoids, proteins, carbohydrates, polyphenolic compounds, tannins, saponins | Ethanol induced liver damage in male rats | ALT, AST, ALP and TB, were significantly decreased | [51] | ||
| Alocasia indica | Ethanolic and aqueous extract of tuber | Flavonoids, alkaloids, glycosides, saponin and tannins | CCl4 induced hepatic injuries in male Albino Wistar rats | Recovery % of serum ALT by 65.32% and AST by 77.36% | [52] | ||
| Antrodia cinnamomea | Aqueous extract and ethanolic extract of fruiting bodies and mycelia | Triterpenoids, benzenoids, diterpenes, steroids, maleic/succinic acid derivatives | CCl4 induced liver injury and ethanol induced liver injury in rats | Suppression of ethanol and CCl4 induced elevation of expression of hepatic mRNAs, i.e. MMP-9, TNFα, KLF-6, and TGFβ1 levels | [53] | ||
| Bidens pilosa | Ethanolic extracts of aerial parts | Flavonoids and polyacetylenes | CCl4 induced liver injury in Male Balbc mice. | Significant decrease serum enzymatic activities of ALT, AST and LDH | [54] | ||
| Boerhavia diffusa | Ethanolic extract of roots | Isoflavonoids, flavonoid glycosides, steroids, alkaloids, and phenolic and lignan glycosides | Hepatotoxicity induced by country made liquor in rats | Reduced the increment in serum parameters like SAP, SGPT, TGs, and total lipid levels | [55] | ||
| Caesalpenia crista | Ethanolic extract of leaves | Carbohydrate, alkaloids, glycosides and phenolic compounds | Paracetamol induced hepato-toxicity in rats. | Reversed the levels of SGOT, SGPT, ALP, TB and TGs | [56] | ||
| Chelidonium majus | Ethanolic extract of whole plant | Benzyl isoquinoline alkaloids viz. protopine, protoberberine, benzophenanthredine | p-dimethyl aminoazoben-zene (p-DAB) induced hepatocarcinogenesis in mice | Biochemical assay of marker enzymes and histology of liver sections suggest hepa-toprotective effects | [57] | ||
| Cyperus rotundus | Methanolic extract of leaves | Flavonoids and alkaloids | CCl4 induced liver damage in wistar albino rats | Lowering the serum levels of various biochemical parameters such as serum SGPT, SGOT, ALP | [58] | ||
| Dendrophthoe falcate | Aqueous and ethanolic extracts of leaves | Flavonoids, phenols | Liver damage was induced by intraperitonial administration of 25% CCl4 in olive oil in rats | Reduced the increment in serum parameters like ALP, AST, ALT, TP and TB | [59] | ||
| Ficus carica | Pet ether extract, aqueous extract and meth-anolicextract of leaves, fruits and roots | Phenolics organic acids and volatile compounds | Liver damage was induced by intraperitonial administration of 25% CCl4 in olive oil in rats | Significant reversal of biochemical, histological, and functional changes | [60] | ||
| Hibiscus rosasinensis | Aqueous extract of flower | Flavonoids, saponins, tannins, phenols, sterols, alkaloids, and anthocyanins | Induced hypercholesterolemi a by feeding pure cholesterol and cholic acid orally mixing with coconut oil in rats | Significant reversal of biochemical, histological and functional changes | [61] | ||
| Leptadenia pyrotechnica | Petroleum ether, chloroform, acetone, methanolic and aqueous extract of whole plant | Flavonoids and polyphenolic compounds | Paracetamol induced liver damage in wistar rats | A marked reduction in the elevated activities of the hepatic enzymes i.e. SGPT, SGOT, ALP and TB levels | [62] | ||
| Loranthus parasiticus | Ethanolic extract of leaves | Sesquiterpene lactones (corianin, coriamyrtin, tutin, and coriatin) | D-galactosamine and CCl4 damage in rat liver cells | 50% inhibition on SGPT | [63] | ||
| Melastoma malabathricum | Methanolic extract of leaves | Flavonoids | Paracetamol induced liver toxicity in rats | Serum liver enzymes ALP, ALT and AST as well as the microscopic observations and microscopic scoring supported the hepato-protective potential | [64] | ||
| Oxalis corniculata | Ethanolic extract of whole plants | Flavonoids, phenols and tocopherols | Paracetamoleinduced hepa-totoxicity in wistar rats | Lowering the serum levels of various biochemical parameters such as SGPT, SGOT and ALP | [65] | ||
| Petroselinum crispum | Aqueous extract of leaves | Flavonoids, phenolic compounds and ascorbic acid | Paracetamole induced hepatotoxicity in wistar rats | Significant decrease in blood glucose, ALP, uric acid, sodium and potassium levels, liver lipid peroxidation and nonenzymatic glycosylation and increase in liver glutathione | [66] | ||
| Rheum palmatum | Ethanolic extract of roots | Anthraquinone derivatives and tannin related compound | CCl4 induced liver injury in rats | Elevation of AST, ALT, HA and laminin (LN) levels were reversed | [67] | ||
| Salvia miltiorrhiza | Ethanolic extract of roots | Salvianolic acids, tanshinone, cryptotanshinone | CCl4 induced liver injury in rats | Induce apoptosis of hepatic stellate cells (HSCs) | [68] | ||
| Tephrosia purpurea | Ethanolic extract of roots | Purpurin, purpurenone, dehydrolsodericin, maackiain, semiglabrin and pseudosemiglabrin | CCl4 induced oxidative damage and resultant dysfunction in the liver of rats. | Induce apoptosis of hepatic stellate cells (HSCs) | [69] | ||
| Terminalia arjuna | Aqueous extract of bark | Flavonoids, tannins and oligomeric proanthocyanidins | Cadmium induced toxicity in Albino Rats | Significantly reversed the elevated the serum levels of following biomarkers AST, ALT, ALP and MDA | [70] | ||
| Trigonella foenum graecum | Ethanolic extract of seeds | β-carotein, saponins, coumarin, nicotinic acid, phytic acid, scopolatin, trigonelline | Thioacetamide induced liver cirrhosis in rats | The elevated levels of alkaline phosphatase, glutamyl transferase and selected biochemical markers of liver cirrhosis were reversed. | [71] | ||
| Ziziphus mucronata | Methanolic extract of leaves | Phenols | Dimethoate induced liver damage in rats | A significant decline serum marker enzymes SGPT, SGOT and ALP | [72] | ||
The hepatoprotective effects of Agrimonia eupatoria water extract (AE) were studied in chronic ethanol induced liver injury in rats. The results revealed that AE ameliorates chronic ethanol-induced liver injury protection is likely due to the suppression of oxidative stress and TLR-mediated inflammatory signaling [20,21].
The ethanolic and aqueous extracts of Anchusa strigosa were studied to inhibit aryl hydrocarbon hydroxylase activity (AHH) and 3H-benzo (a) pyrene (3H-BP) binding to rat liver microsomal protein. The aqueous extracts showed no inhibitory effect, while the ethanolic extracts showed a strong inhibitory effect on both AHH and 3H-BP binding to the microsomal protein [22,23].
Arctium lappa was shown to suppress the CCl4 or acetaminophen-intoxicated mice as well as the ethanol plus CCl4-induced rat liver damage. The underlying hepatoprotective ability of Arctium lappa could be related to the decrease of oxidative stress on hepatocytes by increasing glutathione (GSH), cytochrome P-450 content and NADPH-cytochrome C reductase activity and by decreasing malondialdehyde (MDA) content, hence alleviating the severity of liver damage based on histopathological observations [24–26].
The treatment with an aqueous extract of Brassica rapachinensis significantly combats the oxidative stress imposed by t-BHP in the hepatic tissues as evidenced by a marked improvement in the antioxidant status and suppression of lipid peroxide levels. In addition, the study result indicates that the release of glucose at the 7-position in isorhamnetin 3,7-di-O-glucoside was significant in mitigating liver injury [27].
Esculetin, a phenolic compound found in Cichorium intybus was investigated for its possible protective effect against paracetamol and CCl4-induced hepatic damage. Pretreatment of rats with esculetin (6 mg/kg) prevented the paracetamol-induced rise in serum enzymes. Furthermore, Esculetin also prevented CCl4-induced prolongation in pentobarbital sleeping time, confirming hepatoprotective [28].
Cichorium intybus root extract therapy normalized some morphofunctional liver features (decreases glycogen content and necrosis and increases the number of cells with pronounced protein synthesis activity in rats with CCl4-induced hepatitis [29].
The effect of Cichorium intybus L. seed extract was evaluated in hepatic steatosis caused by early and late-stage diabetes in rats and induced in HepG2 cells (in vitro) by BSA-oleic acid complex (OA). Cichorium intybus seed extract acted as a peroxisome proliferator activated receptor alpha (PPARα) agonist. Cichorium intybus seed extract released glycerol from HepG2 cells and targeted the first and the second hit phases of hepatic steatosis [30,31].
The methanolic extract from fresh stems of Cistanche tubulosa possessed hepatoprotective effects against D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced liver injury in mice. Among the isolated compounds, echinacoside, acteoside, isoacteoside, acetylacteoside, and tubuloside A, inhibited D-GalN-induced death of hepatocytes [32].
The petroleum ether extract of Crotalaria juncea seed at low and high dose (100 and 500 mg/kg) were tested for its efficacy against thioacetamide induced acute hepatic damage in rats. The results proved that the Crotalaria juncea seed extract possessed hepatoprotective potency in a dose-dependent manner by reducing the elevated levels of marker enzymes and increasing the decreased antioxidant enzyme activity [33,34].
The effects of Cyperus rotundus rhizome on cellular lipogenesis and non-alcoholic/diet-induced fatty liver disease and the molecular mechanism of these actions were studied. Study results suggested that the hexane fraction of Cyperus rotundus might be a novel therapeutic remedy for fatty liver disease through the selective inhibition of the lipogenic pathway [35,36].
The protective role of Datura stramonium leaves ethanolic extract against acute carbaryl toxicity was studied in rats. The animal with a toxic dose of carbaryl showed a mainly cholinergic effect, while those with a toxic dose of Datura stramonium extract showed mainly anticholinergic effect. The isobolographic analysis showed that the sort of interaction was highly antagonistic. There was an increase in the combined LD50 of carbaryl and Datura stramonium extract, nearly double that of each alone. This was due to the high tropane alkaloid contents of Datura stramonium that abolish carbaryl cholinergic toxic effect by blocking the muscarinic receptors of parasympathetic nerve ending [37,38].
The renoprotective activity of Daucus carota root extract was studied in renal ischemia reperfusion injury in rats. Pretreatment with Daucuscarota extracts was associated with a significantly lower malondialdehyde level. Accordingly, Daucus carota extracts exerted renoprotective activity probably by the free radical scavenging activity [39,40].
The hepatoprotective effect of kaempferol (100 and 200 mg/kg bw) isolated from Daucus carota leaves was tested in paracetamol-induced liver damage of albino rats. Paracetamol induced a significant (p < 0.05) increase in liver enzymes along with hepatic necrosis and other visible disarrangements in hepatic tissues. Oral treatment with kaempferol reversed all the serum and liver parameters dose-dependently [41].
The inhibitory effect of crude leaves of Dodonaea viscosa was studied on the lead acetate-induced synthesis of glycoproteins and sialic acid in the liver and plasma. Administration of crude leaves of Dodonaea viscose (100 mg/100 g bw orally) effectively suppressed the synthesis of glycoproteins and sialic acid in the liver and thereby controlling the concentration in plasma. The authors concluded that Dodonaea viscose may exert its membrane protection effect by inhibiting the synthesis of glycoproteins and sialic acid induced by lead acetate [42,43].
The hepatoprotective effects and underlying mechanism of Dolichos lablab water extract (DLL-Ex) were evaluated using an in vitro cellular model in which non-alcoholic fatty liver disease (NAFLD) was simulated by inducing excessive FFA influx into hepatocytes. DLL-Ex significantly attenuated FFA-mediated cellular energy depletion and mitochondrial membrane depolarization. Furthermore, DLL-Ex enhanced phosphorylation of AMPK, indicating that AMPK is a critical regulator of DLL-Ex-mediated inhibition of hepatic lipid accumulation, possibly through its antioxidative effect [44,45].
An aqueous extract of Eupatorium cannabinum exhibited anti-necrotic activity against carbon tetrachloride-induced hep-atotoxicity in rats. The effect is attributed to the presence of flavonoids, rutoside, hyperoside, quercetin, phenolic acids, caffeic and chlorogenic; and not to the presence of eupatoriopicrin. In addition, acrylic acid and the lactic, malic and citric acids present in the plant also exhibited a protective effect against acute toxicity induced by ethanol in mice [46].
The protective effect of Fumaria parviflora on nimesulide-induced cell death was investigated in primary rat hepatocyte cultures. Study results indicated that of Fumaria parviflora extract modulates critical events regulating pro and anti-apoptotic proteins in mitochondria dependent apoptosis induced by nimesulide [47].
A-Hepatica is an herbal combination (contained ten herbs included Geum urbanum (Clove root-6.5 ml) was used for detoxification of the liver and gallbladder. A-Hepatica was said to be regulates secretion and absorption in the digestive system, has anti-inflammatory and antispasmolytic function in the portal vein, stimulates bile flow and increases detoxification of the liver [48].
The polyphenol-rich fraction (WP, 45% polyphenol) prepared from the kernel pellicles of walnuts was assessed for its hepatoprotective effect in mice. A single oral administration of WP (200 mg/kg) significantly suppressed serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) elevation in liver injury induced by carbontetrachloride, while it did not suppress D-Galactosamine (D-GalN)-induced liver injury. However, of the isolated constituents, only ellagitannins with a galloylated glucopyranose core, such as tellimagrandins I, II, and rugosin C, suppressed CCl4-induced hepatocyte damage significantly [49].
Elevation of malondialdehyde (MDA) and glutathione (GSH) levels in liver homogenate (6-and 2-folds, respectively) was significantly reduced by 50% and 41% on treatment with the low dose of Jussiaea repens extract. This was an evidence of the strong antioxidant and consequently hepatoprotective effect of this extract, which could be attributed to its high flavonoid content [50].
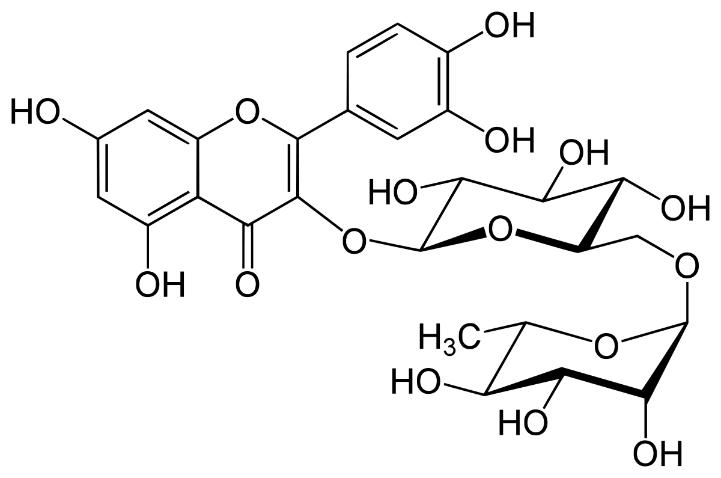
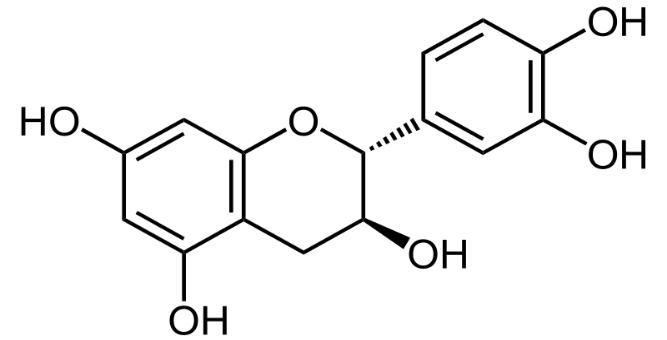
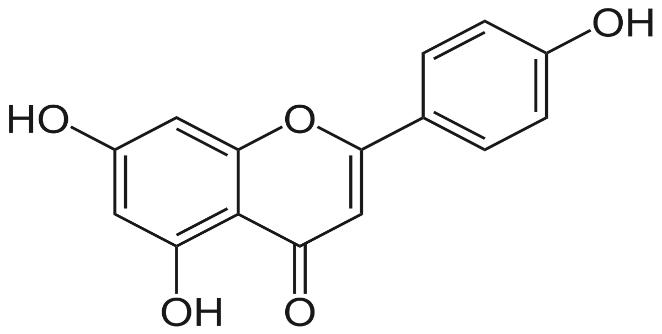
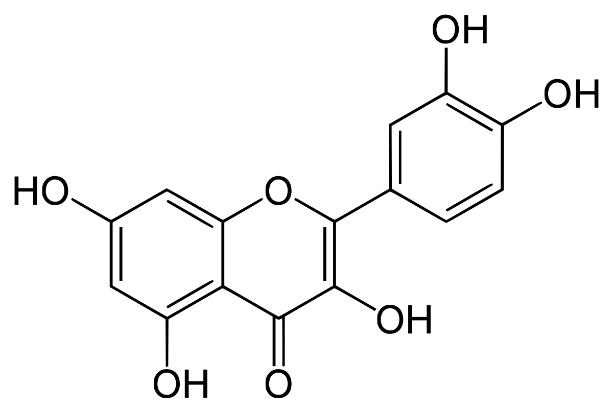
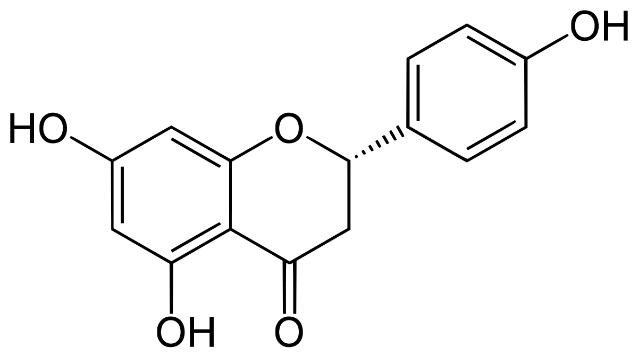
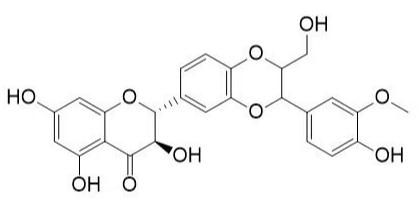
Several clinical investigations have shown flavonoids efficacy and safety in treating hepatobiliary dysfunction and digestive complaints, such as the sensation of fullness, loss of appetite, nausea, and abdominal pain. Flavonoids such as rutin, catechin, apigenin, quercetin, naringenin, and venoruton are reported for their hapatoprotective activities (Figures 2–6) [8].
Different chronic ailments such as diabetes may lead to progress of hepatic clinical manifestations. Glutamate-cysteine ligase catalytic subunit (Gclc) expression, glutathione, and ROS levels are reported to be decreased in the liver of diabetic mice. Anthocyanins have drawn increasing attention for their preventive effect against various diseases. Silymarin (Figure 7) is a flavonoid having three structural components silydianine, silibinin, and silychristine extracted from the seeds and fruit of milk thistle Silybum marianum (Compositae). Silymarin has been reported to stimulate the enzymatic activity of DNA-dependent RNA polymerase 1 and subsequent biosynthesis of RNA and protein, resulting in DNA biosynthesis and cell proliferation prominent to liver regeneration only in damaged livers [73]. Silymarin increases proliferating hepatocytes in response to Fumonisin B1 (FB1, a mycotoxin produced by Fusarium verticillioides) prompted cell death without modulation of cell proliferation in normal livers. The pharmacological properties of silymarin involve the regulation of cell membrane permeability and integrity, inhibition of leukotriene, ROS scavenging, suppression of NF-kB activity, depression of protein kinases, and collagen production [74]. Silymarin has clinical applications in treating cirrhosis, ischemic injury, and toxic hepatitis induced by various toxins such as acetaminophen and mushroom [75].
FUTURE PROSPECTS
Prevention and cure of diseases using phytochemicals, especially flavonoids are well known. The structure function relationship of flavonoids is epitome of major biological activities. The future of phytopharmaceuticals is bright as it undoubtedly serves as a cheap and steady variety of therapeutic agents of great significance in the health care of mankind. Therefore attention is drawn to the potential of medicinal plants that have the hepatoprotective ability to reduce or cure liver disorders. In general, natural drug substances offer vital and appreciable roles in the modern system of medicine, thereby adequately justifying their legitimate presence in the prevailing therapeutic arsenal. It has been seen that herbal hepatoprotective drugs have fewer side effects or interactions compared to synthetic medicine. However, scientific evidence from tests done to evaluate the safety and effectiveness of traditional hepatoprotective medicine products and practices is limited and further study of products and practices is needed. Pharmacokinetic and toxicity studies have not disclosed any issues that could limit the therapeutic use of these drugs. The structure function relationship of flavonoids is epitome of major biological activities. With genetic modifications, it is now possible to produce flavonoids on a large scale. Additional achievements will provide newer insights and will undoubtedly lead to a new era of flavonoid based pharmaceutical agents for treating many infectious and degenerative diseases. Further studies including clinical trials need to be carried out to ascertain the safety of these compounds as a good alternative to conventional drugs in the treatment of liver diseases.
CONCLUSION
Herbal medications have shown the ability to maintain the normal functional status of the liver with or without fewer side effects; that’s why herbal hepatoprotectives are preferred mainly by medical practitioners, as well as over-the-counter. Despite tremendous advances in modern medicine, the hepatic disease remains a worldwide health problem; thus, the search for new drugs is still ongoing. Numerous formulations of medicinal plants are used to treat liver disorders and many of these treatments act as radical scavengers, whereas others are enzyme inhibitors or mitogens. The hepatoprotective activity of the plants is probably due to the presence of flavonoids and fractions or mixture extracts of plants. Plant drugs (combinations or individual drug) for liver diseases should possess sufficient efficacy to cure severe liver diseases caused by toxic chemicals, viruses, alcohol intake, and repeated administration of drugs like paracetamol, rifampicin and isoniazid. A single medication cannot be effective against all types of severe liver diseases. Effective formulations must be developed using indigenous medicinal plants, with good pharmacological experiments and clinical trials. Standards of safety and efficacy should govern the manufacture of plant products.
Conflict of Interest
The author declares no conflicts of interest.
Author’s Contribution
The author Gaurav Dubey confirms sole responsibility for the conception and design of the study, data collection, analysis and interpretation of results, and manuscript preparation.
REFERENCES
| [1] | Saleem TS, Chetty SM, Ramkanth S, Rajan VST, Kumar KM, Gauthaman K. Hepatoprotective herbs: a review. Int J Res Pharma Sci. 2010;1:1–5. |
| [2] | Zhang A, Sun H, Wang X. Recent advances in natural products from plants for the treatment of liver diseases. Eur J Med Chem. 2013;63:570–577. Doi: 10.1016/j.ejmech.2012.12.062. |
| [3] | Cowart LA. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol. Metab. 2009;20:34–42. Doi: 10.1016/j.tem.2008.09.004. |
| [4] | Michel F, Mas E, Drai J. Biomarkers of lipid peroxidation: analytical aspects. Ann Biol Clin. 2008;66:605–620. Doi: 10.1684/abc.2008.0283. |
| [5] | Orhan DD, Orhan N, Ergun E, Ergun F. Hepatoprotective effect of Vitisvinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats. J Ethnopharmacol. 2007;112(1):145–151. Doi: 10.1016/j.jep.2007.02.013. |
| [6] | Khanam UKS, Oba S, Yanase E, Murakami Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Functional Foods. 2012;4:979–987. Doi: 10.1016/j.jff.2012.07.006. |
| [7] | Arem AEI, Ghrairi F, Lahouar L, Thouri A, Saafi EB, Ayed A, et al. Hepatoprotective activity of date fruit extracts against dichloro acetic acid induced liver damage in rats. J Functional Foods. 2014;9:119–130. Doi: 10.1016/J.JFF.2014.04.018. |
| [8] | Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as nutraceuticals: a review. Tropical J Pharma Res. 2008;7:1089–1099. Doi: 10.4314/tjpr.v7i3.14693. |
| [9] | Chaudhari NB, Chittam KP, Patil VR. Hepatoprotective activity of Cassia fistula seeds against paracetamol-induced hepatic injury in rats. Arch Pharm Sci & Res. 2009;1:218–21. |
| [10] | Evans WC. Trease and Evans pharmacognosy. 15th ed. Philadephia: Elsevier Science Limited; 2002. pp.414–415. |
| [11] | Williamson EM, Okpako DT, Evans FJ. Selection, evaluation and pharmacological evaluation of plant material. England: John Wiley and Sons; 1996.47–51. |
| [12] | Wu HM, Yang YM, Kim SG. Rimonabant, a cannabinoid receptor type 1 inverse agonist, inhibits hepatocyte lipogenesis by activating liver kinase B1 and AMP-activated protein kinase axis downstream of Gα i/o inhibition. Mol Pharmacol. 2011;80(5):859–869. Doi: 10.1124/mol.111.072769. |
| [13] | Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis-a randomised clinical trial. J Hepatol. 2006;44(4):784–790. Doi: 10.1016/j.jhep.2005.11.039. |
| [14] | O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14–32. Doi: 10.1038/ajg.2009.593. |
| [15] | Domitrovic R, Potocnjak I. A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch Toxicol. 2016;90(1):39–79. Doi: 10.1007/s00204-015-1580-z. |
| [16] | Tai M, Zhang J, Song S, Miao R, Liu S, Pang Q, et al. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int Immunopharmacol. 2015;27(1):164–170. Doi: 10.1016/j.intimp.2015.05.009. |
| [17] | Domitrovic R, Jakovac H, Grebic D, Milin C, Radosevic-Stasic B. Dose and time dependent effects of luteolin on liver metallothioneins and metals in carbon tetrachloride-induced hepatotoxicity in mice. Biol Trace Elem Res. 2008; 126(1–3):176–185. Doi: 10.1007/s12011-008-8181-0. |
| [18] | Domitrovic R, Jakovac H, Milin C, Radosevic-Stasic B. Dose and time dependent effects of luteolin on carbon tetrachloride-induced hepatotoxicity in mice. Exp Toxicol Pathol. 2009;61(6):581–589. Doi: 10.1016/j.etp.2008.12.005. |
| [19] | Domitrovic R, Jakovac H, Tomac J, Sain I. Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol Appl Pharmacol. 2009;241(3):311–321. Doi: 10.1016/j.taap.2009.09.001. |
| [20] | Al-Snafi AE. The pharmacological and therapeutic importance of Agrimonia eupatoria- A review. Asian J Pharm Sci Technol. 2015;5(2):112–117. |
| [21] | Yoon SJ, Koh EJ, Kim CS, Zee OP, Kwak JH, Jeong WJ, et al. Agrimonia eupatoria protects against chronic ethanol-induced liver injury in rats. Food Chem Toxicol. 2012;50(7):2335–2341. Doi: 10.1016/j.fct.2012.04.005. |
| [22] | Alwan AH, Al-Gaillany KAS, Naji A. Inhibition of the binding of 3H-Benzo [a] pyrene to rat liver microsomal protein by plant extracts. Int J Crude Drug Res. 1989;27:33–37. Doi: 10.3109/13880208909053935. |
| [23] | Al-Snafi AE. The pharmacology of Anchusa italica and Anchusa strigosa – a review. Int J Pharm Pharm Sci. 2014;6(4):7–10. |
| [24] | Lin SC, Chung TC, Lin CC, Ueng TH, Lin YH, Lin SY, et al. Hepatoprotective effects of Arctium lappa on carbon tetrachloride- and acetaminophen-induced liver damage. Am J Chin Med. 2000;28(2):163–173. Doi: 10.1142/S0192415X00000210. |
| [25] | Lin SC, Lin CH, Lin CC, Lin YH, Chen CF, Chen IC, et al. Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J Biomed Sci. 2002;9(5):401–409. Doi: 10.1007/BF02256533. |
| [26] | Al-Snafi AE. The pharmacological importance and chemical constituents of Arctium lappa. a review. Int J Pharm Res Scholars. 2014;3(1–1): 663–670. |
| [27] | Li L, Park DH, Li YC, Park SK, Lee YL, Choi HM, et al. Anti-hepatofibrogenic effect of turnip water extract on thioacet-amide-induced liver fibrosis. Lab Anim Res. 2010;26(1):1–6. Doi: 10.5625/lar.2010.26.1.1. |
| [28] | Zafar R, Mujahid Ali S. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J Ethnopharmacol. 1998;63(3):227–231. Doi: 10.1016/s0378-8741(98)00087-7. |
| [29] | Ahmed B, Khan S, Masood MH, Siddique AH. Anti-hepatotoxic activity of cichotyboside, a sesquiterpene glycoside from the seeds of Cichorium intybus. J Asian Nat Prod Res. 2008; 10(3–4):223–231. Doi: 10.1080/10286020701590764. |
| [30] | Heibatollah S, Reza NM; Izadpanah G, Sohailla S. Hepatoprotective effect of Cichorium intybus on CCl4-induced liver damage in rats. African J Biochem Res. 2008; 2(6):141–144. |
| [31] | Ziamajidi N, Khaghani S, Hassanzadeh G, Vardasbi S, Ahmadian S, Nowrouzi A, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1. Food Chem Toxicol. 2013;58:198–209. Doi: 10.1016/j.fct.2013.04.018. |
| [32] | Al-Snafi AE. Medical importance of Cichorium intybus — a review. IOSR J Phar. 2016; 6(3): 41–56. |
| [33] | Lin JK, Wang CJ. Protection of crocin dyes on the acute hepatic damage induced by aflatoxin B1 and dimethyln-trosamine in rats. Carcinogenesis. 1986;7(4):595–599. Doi: 10.1093/carcin/7.4.595. PMID: 2870820. |
| [34] | Rahila KC, Bhatt L, Chakraborty M, Kamath JV. Hepatoprotective activity of Crotalaria juncea against thioacetamide intoxicated rats. Int Res J Pharm App Sci. 2013;3(1):98–101. |
| [35] | Jain A, Jain IP, Singh SP, Agrawal A. To evaluate hepatoprotective activity of roots of Cynodon dactylon- an experimental study. Asian J Pharm Clin Res. 2013; 6(2):109–112. |
| [36] | Al-Snafi AE. A review on Cyperus rotundus: a potential medicinal plant. IOSR J Phar. 2016;6(7):32–48. |
| [37] | Oh GS, Yoon J, Lee GG, Kwak JH, Kim SW. The hexane fraction of Cyperus rotundus prevents non-alcoholic fatty liver disease through the inhibition of liver x receptor α-mediated activation of sterol regulatory element binding protein-1c. Am J Chin Med. 2015;43(3):477–494. Doi: 10.1142/S0192415X15500305. |
| [38] | Bania TC, Chu J, Bailes D, O’Neill M. Jimson weed extract as a protective agent in severe organophosphate toxicity. Acad Emerg Med. 2004;11(4):335–338. Doi: 10.1197/j.aem.2003.12.002. PMID: 15064204. |
| [39] | Mahmood KA, Abbas DA. The protective role of Datura stramonium leaves ethanolic extract against acute carbaryl toxicity in rats. Int J Biomed Adv Res. 2015;6(05):400–405. Doi: 10.7439/IJBAR. |
| [40] | Al-Snafi AE. Medical importance of Datura fastuosa (syn: Daturametel) and Datura stramonium - a review. IOSR J Pharm. 2017;7(2):43–58. |
| [41] | Singh K, Singh N, Chandy A, Manigauha A. In vivo antioxidant and hepatoprotective activity of methanolic extracts of Daucus carota seeds in experimental animals. Asian Pac J Trop Biomed. 2012;2(5):385–388. Doi: 10.1016/S2221-1691(12)60061-6. |
| [42] | Wang JKT, Portbury S, Thomas MB, Barney S, Ricca DJ, Morris DL, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci U S A. 2006;103(27):10461–10466. Doi: 10.1073/pnas.0600930103. |
| [43] | Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, et al. Ouabain triggers preconditioning through activation of the Na+, K+-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73(3):488–496. Doi: 10.1016/j.cardiores.2006.11.003. |
| [44] | Sivanesan D, Veera AV, Selvi T. Protective effect of Dodonaea viscosa (L) against lead acetate induced altered glycoprotein profiles in rats. J Chem. 2009; 6(3):725–728. |
| [45] | Al-Snafi AE. A review on Dodonaea viscosa: A potential medicinal plant. IOSR J Pharm. 2017;7(2):10–21. |
| [46] | Lexa A, Fleurentin J, Lehr PR, Mortier F, Pruvost M, Pelt JM. Choleretic and hepatoprotective properties of Eupatorium cannabinum in the rat. Planta Med. 1989;55(2):127–132. Doi: 10.1055/s-2006-961904. |
| [47] | Sharma UR, Prakash T, Surendra V, Roopakarki N, Goli D. Hepatoprotective activity of Fumaria officinalis against CCl4-induced liver damage in rats. Pharmacologia 2012;3(1):9–14. Doi: 10.5567/pharmacologia.2012.9.14. |
| [48] | Khan MA, Shafiullah J, Malik SA, Shafi M. Hepatoprotective effects of Berberis lycium, Galiuma parine and Pistacia integerrima in carbon tetrachloride (CCl4) treated rats. J Post Grad Med Inst. 2008;22(2):91–94. |
| [49] | Benhouda A, Mouloud Y, Hachani K, Nassiba C, Hadjar B, Souhila B, et al. In vivo evaluation of hepatoprotective, anti-asthmatic and antiallergic activities of methanolic extracts of leaves of Hyoscyamus albus L. (solanaceae) and Umbilicus rupestris L. (crassulaceae). The 15th International Congress of the International Society for Ethno-Pharmacology, Shoubak University College, 2015:3. |
| [50] | Nabavi SF, Ebrahimzadeh MA, Nabavi SM, Mahmoudi M, Rad SK. Biological activities of Juglans regia flowers. Braz J Pharmacogn. 2011;21(3):465–470. |
| [51] | Kumar KE, Harsha KN, Sudheer V, Babu NG, In vitro anti-oxidant activity and in vivo hepatoprotective activity of aqueous extract of Allium cepa bulb in ethanol induced liver damage in Wistar rats. Food Sci and Hum Wellness. 2013;2:132–138. Doi: 10.1016/j.fshw.2013.10.001. |
| [52] | Pal S, Bhattacharjee A, Mukherjee S, Bhattacharya K, Khowala S. Antioxidant and hepatoprotective activity of ethanolic extract of Alocasia indica tuber. American J Phytomed Clin Therap. 2014;2:191–208. |
| [53] | Liu YW, Lu KH, Ho CT, Sheen LY. Protective effects of Antrodia cinnamomea against liver injury. J Tradit Complement Med. 2012;2(4):284–294. Doi: 10.1016/s2225-4110(16)30114-6. |
| [54] | Kviecinski MR, Felipe KB, Correia JF, Ferreira EA, Rossi MH, de Moura Gatti F, et al. Brazilian Bidens pilosa Linne yields fraction containing quercetin-derived flavonoid with free radical scavenger activity and hepatoprotective effects. Libyan J Med. 2011;6(1):1–8. Doi: 10.3402/ljm.v6i0.5651. |
| [55] | Mishra G, Khosa RT, Singh P, Jha KK. Hepatoprotective potential of ethanolic extract of Caesalpenia crista leaves against paracetamol induced hepatotoxicity in rats. J Coast Life Med. 2014;2:575–579. Doi: 10.12980/JCLM.3.2015JCLM-2014-0036. |
| [56] | Mishra S, Aeri V, Gaur PK, Jachak SM. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. Biomed Res Int. 2014;2014:808302. Doi: 10.1155/2014/808302. |
| [57] | Biswas SJ, Bhattacharjee N, Khuda-Bukhsh AR. Efficacy of a plant extract (Chelidonium majus L.) in combating induced hepatocarcinogenesis in mice. Food Chem Toxicol. 2008;46(5):1474–1487. Doi: 10.1016/j.fct.2007.12.009. |
| [58] | Rao YN, Rao MP, Rao VR, Suresh K, Rafi M, Rao TN, et al. Prelimeinary phytochemical screening and hepatoprotective activity of methanolic leaves extract of Cyperus rotundus in CCl4 induced hepetotoxicity in albino Wistar rats. World J Pharm Pharm Sci. 2014;3:787–795. |
| [59] | Haque A, Tahmina, Afsana SK, Sarker IR, Hossain M, Islam S, et al. Antioxidant and hepatoprotective effects of aqueous and ethanol extracts of Dendrophthoe falcate Linn leaves. Archives 2014;1:90–101. |
| [60] | Mawa S, Husain K, Jantan I. Ficus carica L. (Moraceae): phytochemistry, traditional uses and biological activities. Evid Based Complement Alternat Med. 2013;2013:974256. Doi: 10.1155/2013/974256. |
| [61] | Biswas A, D’Souza UJA, Bhat S, Damodar D. The hepatoprotective effect of Hibiscus rosasinensis flower extract on diet-induced hypercholesterolemia in male albino wister rats. Int J Med Pharm Sci. 2014;04(06):1–5. |
| [62] | Partap S, Tewari U, Sharma K, Jha KK. Hepatoprotective activity of whole plant extract of Leptadenia pyrotechnica against paracetamol induced damage in rats. J Drug Delivery Ther. 2014;4:36–39. Doi: 10.22270/jddt.v4i1.743. |
| [63] | Moghadamtousi SZ, Kamarudin MN, Chan CK, Goh BH, Kadir HA. Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am J Chin Med. 2014;42(1):23–35. Doi: 10.1142/S0192415X14500025. |
| [64] | Mamat SS, Kamarolzaman MF, Yahya F, Mahmood ND, Shahril MS, Jakius KF, et al. Methanol extract of Melastoma malabathricum leaves exerted antioxidant and liver protective activity in rats. BMC Complement Altern Med. 2013;13:326. Doi: 10.1186/1472-6882-13-326. |
| [65] | Sreejith G, Jayasree M, Latha PG, Suja SR, Shyamal S, Shine VJ, et al. Hepatoprotective activity of Oxalis corniculata L. ethanolic extract against paracetamol induced hepatotoxicity in Wistar rats and its in vitro antioxidant effects. Indian J Exp Biol. 2014;52(2):147–152. |
| [66] | Farzaei MH, Abbasabadi Z, Ardekani MR, Rahimi R, Farzaei F. Parsley: a review of ethnopharmacology, phytochemistry and biological activities. J Tradit Chin Med. 2013;33(6):815–826. Doi: 10.1016/s0254-6272(14)60018-2. |
| [67] | Wang BQ. Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J Med Plant Res. 2010;4:2813–2820. |
| [68] | Parajuli DR, Park EJ, Che XH, Jiang WY, Kim YC, Sohn DH, et al. PF2401-SF, standardized fraction of Salvia miltiorrhiza, induces apoptosis of activated hepatic stellate cells in vitro and in vivo. Molecules. 2013;18(2):2122–2134. Doi: 10.3390/molecules18022122. |
| [69] | Dalwadi PP, Patel JL, Patani PV. Tephrosia purpurea Linn (Sharpunkha, Wild Indigo): A review on phytochemistry and pharmacological studies. Indian J Pharm Biol Res. 2014;2:108–121. Doi: 10.30750/ijpbr.2.1.18. |
| [70] | Haidry MT, Malik A. Hepatoprotectiveand antioxidative effects of Terminalia arjuna against cadmium provoked toxicity in albino rats (Ratus Norvigicus). Biochem Pharmacol. 2014;3:130. Doi: 10.4172/2167-0501.1000130. |
| [71] | Zargar S. Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi J Biol Sci. 2014;21:139–145. Doi: 10.1016/j.sjbs.2013.09.002. |
| [72] | Kwape TE, Chaturvedi P, Kamau JM, George S. Hepatoprotective potential of methanol extract of leaf of Ziziphus mucronata (ZMLM) against dimethoate toxicity: biochemical and histological approach. Ghana Med J. 2013;47(3):112–120. |
| [73] | Sonnenbichler J, Zetl I. Biochemical effects of the flavonolignan silibinin on RNA, protein and DNA synthesis in rat livers. In: Progress in Clinical and Biological Research, V. Cody, E. Middleton, and J. B. Karborne, Eds., vol. 213, pp. 319–331, Alan R. Liss, New York, NY, USA, 1986. |
| [74] | He Q, Kim J, Sharma RP. Silymarin protects against liver damage in BALB/c mice exposed to fumonisin B1 despite increasing accumulation of free sphingoid bases. Toxicol Sci. 2004;80(2):335–342. Doi: 10.1093/toxsci/kfh148. |
| [75] | Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–2063. Doi: 10.2165/00003495-200161140-00003. |