Chitosan and Its Derivatives: A Versatile Biopolymer for Diverse Applications
Volume 1, Issue 1, Pages 39-44
Abstract
Chitosan, a linear polysaccharide derived from the deacetylation of chitin, is a highly favourable biopolymer due to its distinctive properties, including biodegradability, biocompatibility, and antimicrobial activity. Tracked primarily from the exoskeletons of crustaceans, it is a sustainable and ample material. This review article provides a comprehensive overview of chitosan and its various derivatives, focusing on their synthesis, characterization, and wide-ranging applications. We delve into the methods of chemical modification that enhance chitosan’s properties, such as its solubility and functional group availability. Key derivatives like carboxymethyl chitosan (CMC), quaternized chitosan (QCS), and chitosan oligosaccharides (COS) are discussed in detail, highlighting how their tailored properties make them suitable for specific uses in the biomedical, environmental, and food sectors. The article also discourses the current challenges in chitosan research, such as production variability and cost, and frameworks future perspectives aimed at unlocking its full impending through advanced functionalization techniques and novel applications.
Author
Corresponding Author
Rajkumari Thagele
History
Received 16 November 2025
Revised 26 November 2025
Accepted 06 December 2025
Keywords
Chitosan, Degree of acetylation, Polysaccharide, Biopolymer, Carboxymethyl chitosan, Quaternized chitosan, Chitosan oligosaccharides.
Open Access
This is an open access article under the CC BY license https://creativecommons.org/licenses/by/4.0/.
INTRODUCTION
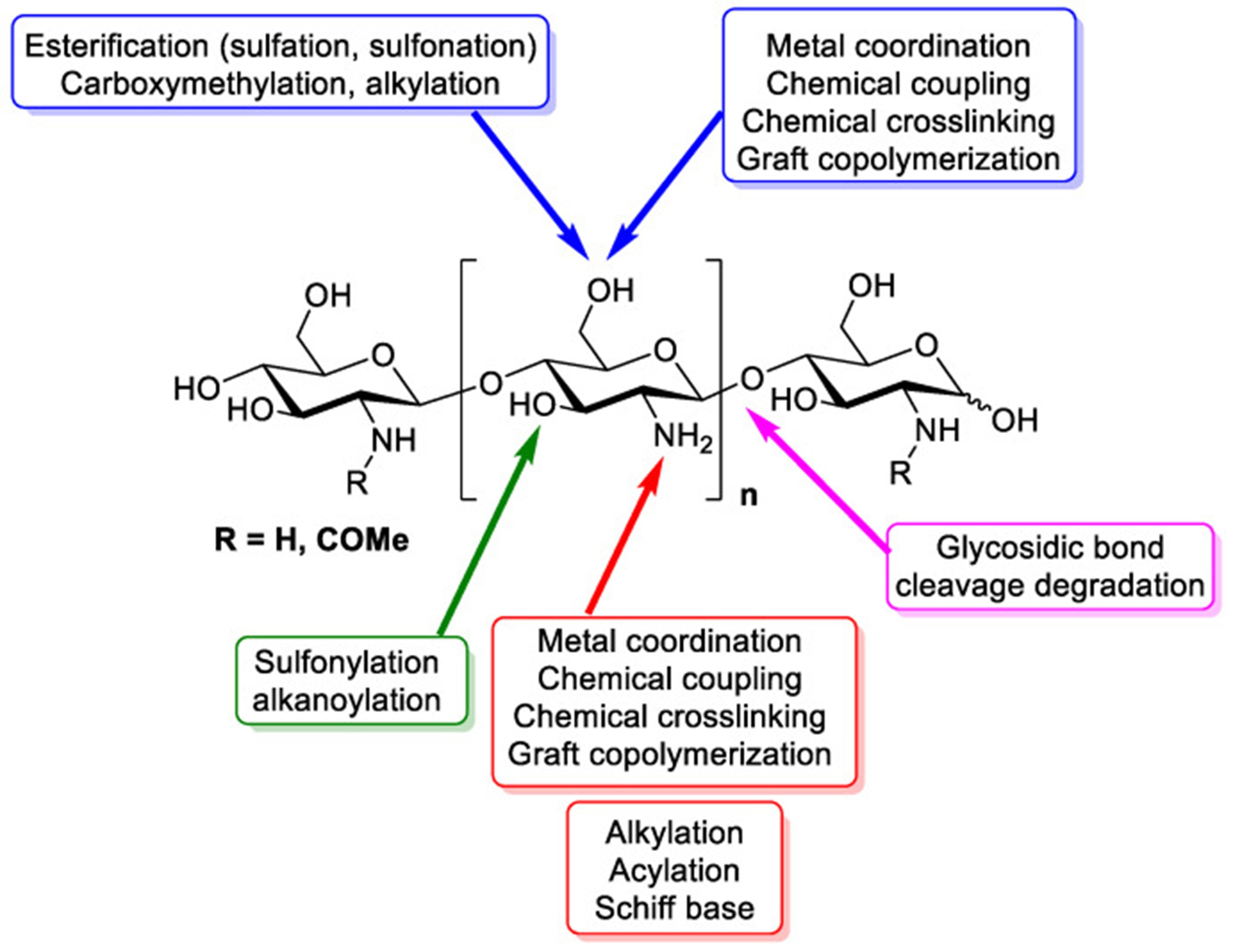
Chitin is one of the furthermost abundant biopolymers on Earth, second only to cellulose, and is a major component of the exoskeletons of arthropods like crabs and shrimp, as well as the cell walls of fungi. Chitosan is a partially deacetylated form of chitin, containing a blend of β-(1→4) bonds linked D−glucosamine and N−acetyl−D−glucosamine units. The degree of deacetylation (DD) is a critical parameter that basically determines its physicochemical properties, such as solubility and charge density. The presence of primary amine groups (- {NH}) on the chitosan backbone gives it a idiosyncratic positive charge in acidic media (pH < 6.5) due to the protonation of these groups (Figure 1). This cationic nature is the key to many of its biological and chemical functionalities, including its antimicrobial properties and ability to form complexes with negatively charged molecules [1 and 2].
Figure 1 - Functional groups in chitosan’s structure [3].
Despite its many advantages, native chitosan has a significant drawback: its poor solubility in neutral and alkaline conditions. This limitation has spurred extensive research into chemical modification, leading to a vast array of chitosan derivatives. These modifications aim to improve solubility, introduce new functionalities, and finetune the material for specific applications. This review reconnoitres the synthesis and properties of chitosan, the most significant derivatives, and their roles in various industries, from medicine to environmental science.
METHODOLOGY
Literature search for the review was conducted (up to December 2025), using Elsevier-Science direct, Springer Link, Wiley Interscience (Wiley), Pubmed and Google Scholar. The search included the following keywords: Chitosan, degree of acetylation, carboxymethyl chitosan, quaternized chitosan, chitosan oligosaccharides. This comprehensive review traces the historical development of chitosan based materials and delves into their specific applications across various fields.
SYNTHESIS AND FUNDAMENTAL PROPERTIES OF CHITOSAN
Production of Chitosan from Chitin
The industrial production of chitosan is a multi-step process that begins with the raw chitinous biomass, typically from shrimp or crab shells. The process involves:
Demineralization: The raw shells are treated with dilute acids (e.g., HCl) to remove inorganic minerals, primarily calcium carbonate.
Deproteinization: The demineralized material is then treated with a dilute alkaline solution (e.g., NaOH) to remove proteins.
Deacetylation: This is the final and most crucial step, where the chitin is treated with a concentrated alkali (e.g., 40–50% NaOH) at high temperatures (100°C). This process removes the acetyl groups from the N-acetyl-D-glucosamine units, converting chitin into chitosan. The extent of deacetylation can be controlled by varying the reaction time, temperature, and alkali concentration [4 and 5].
Physicochemical Properties
The functionality of chitosan is primarily governed by its molecular weight (MW) and degree of deacetylation (DD).
Solubility: Chitosan is insoluble in water, but its amine groups become protonated in acidic solutions, making it soluble. This pH-dependent solubility is a defining characteristic.
Biocompatibility and biodegradability: Chitosan is non-toxic and biocompatible, meaning it is well-tolerated by biological systems. It is also biodegradable, breaking down into non-toxic oligosaccharides through enzymatic action by lysozyme, which is present in the human body.
Antimicrobial activity: The positively charged amine groups on the chitosan chain interact with the negatively charged components of microbial cell membranes, disrupting their integrity and leading to cell death. This property makes it a natural antimicrobial agent [6].
KEY CHITOSAN DERIVATIVES AND THEIR SYNTHESIS
Chemical modifications of chitosan typically target the hydroxyl (−OH) and amine (−NH2) groups, resulting in derivatives with enhanced or new properties [6].
Carboxymethyl Chitosan (CMC)
CMC is a highly water-soluble derivative synthesized by reacting chitosan with monochloroacetic acid in an alkaline medium. The introduction of carboxymethyl groups (−CH2COOH) provides a negative charge, allowing CMC to be soluble over a broad pH range, including neutral and alkaline conditions. This derivative is often used in drug delivery and hydrogel formation.
Quaternized Chitosan (QCS)
QCS is a derivative where quaternary ammonium groups are grafted onto the chitosan backbone, typically by reaction with glycidyl trimethylammonium chloride (GTMAC). This modification creates a permanently cationic polymer, independent of pH. QCS exhibits superior antimicrobial activity compared to native chitosan and is widely used in water purification and wound dressings.
Chitosan Oligosaccharides (COS)
COS are low molecular weight derivatives obtained by the enzymatic or chemical hydrolysis of chitosan. Their small size makes them highly water-soluble and easily absorbable by the body. COS possess a range of biological activities, including antioxidant, anti-tumor, and immunomodulatory effects, making them valuable in the nutraceutical and pharmaceutical industries.
Glycol Chitosan (GC)
Glycol chitosan is synthesized by reacting chitosan with ethylene oxide. The resulting polymer is highly water-soluble, even in physiological pH, which is a major advantage for biomedical applications. GC is commonly used as a scaffold for tissue engineering and as a non-viral vector for gene delivery due to its ability to form stable complexes with nucleic acids [5 and 6].
The unique properties of chitosan and its derivatives have led to their application in a variety of fields.
DIVERSE APPLICATIONS OF CHITOSAN AND ITS DERIVATIVES
The unique properties of chitosan and its derivatives have led to their application in a variety of fields (Table 1).
| S. no. | Carrier system | Active compound | Key findings | References |
|---|---|---|---|---|
| 1. | Nanoparticles | Docetaxel | The nanoparticles components were non-toxic and safe to human cells. The prepared nanoparticles may be used as effective carriers for chemotherapeutic agents targeting carcinogenetic tissues. | [10] |
| 2. | Nanoparticles | Isolongifolene | Isolongifolene-loaded chitosan nanoparticles were shown to be plasma-compatible and to have a consistent release pattern. As a result, chitosan-loaded nanoparticles might be used as an effective adjuvant in cancer therapy to address multi-drug resistance in solid tumours. | [11] |
| 3. | Nanoparticles | Methotrexate | Methotrexate-loaded nanoparticles inhibited the viability of breast cancer cells when exposed to low doses. The tiny size of this efficient cytotoxic nanoparticle delivery method makes it a promising technique for use in breast cancer. | [12] |
| 4. | Nanofibre | Cisplatin | The prepared nanofibres are biocompatible and effective in cancer treatment. | [13] |
| 5. | Nanoparticles | Docetaxel and curcumin | The prepared nanoparticle loaded with curcumin and docetaxel can ameliorate the immunosuppressive microenvironment to promote the inhibition of tumour growth. | [14] |
| 6. | Nanoparticles | Quercetin | The prepared nanoparticles decrease tumour growth and provide a prospective strategy for the treatment of paclitaxel-resistant lung cancer. | [15] |
| 7. | Nanoparticles | Doxorubicin | At pH 5.0, the drug was promptly and totally liberated from the nanoparticles. The anti-HER2 conjugated O-succinyl chitosan graft Pluronic® F127 copolymer nanoparticles had the lowest IC50 in vitro, indicating a boost in the therapeutic effectiveness of doxorubicin to treat human breast cancer. | [16] |
| 8. | Hydrogel nano composite | Curcumin | The proposed nanostructure is a promising vehicle with the ability to improve curcumin loading and enable prolonged curcumin release while causing considerable cytotoxicity in MCF-7 cells. | [17] |
| 9. | Hybrid nanogel containing gold nanoparticles | Doxorubicin | The present research demonstrated that the designed enzyme-responsive nanogel could potentially target various solid tumours both actively and passively. | [18] |
| 10. | Nanoparticles | Doxorubicin and Cisplatin | In human breast cancer MCF-7 cells, doxorubicin plus cisplatin had a synergistic cell-killing impact. The findings clearly show that the innovative nanoplatform holds great promise for synergistic combination treatment of breast cancer. | [19] |
Biomedical Field
Drug delivery: Chitosan and its derivatives are excellent candidates for drug delivery systems due to their mucoadhesive properties and ability to form nanoparticles. They can encapsulate drugs, protect them from degradation, and enhance their bioavailability, particularly in oral and nasal administration [7].
Tissue engineering and regenerative medicine: Chitosan-based scaffolds and hydrogels provide a biocompatible and bio-degradable framework for cell proliferation and differentiation. They are extensively researched for regenerating bone, cartilage, and skin tissues [8].
Wound healing: Chitosan-based wound dressings promote hemostasis, reduce inflammation, and exhibit antimicrobial effects, thereby accelerating the healing process [9].
Environmental Applications
Water treatment: The cationic nature of chitosan makes it an effective flocculant, capable of binding to negatively charged colloidal particles, heavy metals, and organic pollutants in wastewater. QCS, with its permanent positive charge, is particularly efficient for these purposes.
Bioremediation: Chitosan-based adsorbents are used to remove a variety of contaminants, including dyes, pesticides, and other toxins from polluted water and soil [20].
Food and Agriculture
Food preservation: Chitosan-based edible coatings and films extend the shelf life of fruits and vegetables by acting as a barrier against moisture loss and microbial spoilage.
Food additives: Chitosan oligosaccharides (COS) are used as functional food ingredients due to their prebiotic and antioxidant properties.
Agriculture: Chitosan can be used as a natural biopesticide and a growth promoter for crops, helping to improve plant health and yield [3].
CHALLENGES AND FUTURE PERSPECTIVES
Despite the extensive research and promising applications, the widespread commercialization of chitosan and its derivatives faces several challenges. The variability in the properties of the raw material from different sources can affect the final product's quality. Additionally, the high cost of production and the challenges in scaling up some modification processes remain significant hurdles.
Future research should focus on developing more cost-effective and sustainable production methods. Exploring advanced (Table 2) functionalization techniques, such as click chemistry and enzymatic modifications, will allow for the synthesis of new derivatives with highly precise and tunable properties. The integration of chitosan with other materials to create composite materials with enhanced mechanical and functional properties also represents a promising avenue. Ultimately, the development of intelligent, stimuli-responsive chitosan-based materials for biosensors, drug delivery systems, and regenerative medicine will be at the forefront of future innovation.
| Device type | Model drug/drug | Polymer formulation | Effect/Results | References |
|---|---|---|---|---|
| Solid–lipid nanoparticles | Leflunomide | Chitosan/Folic acid | FA-CS-SLNs exhibited sustained release for 168 h and lowered liver toxicity and enhanced joint healing compared to leflunomide suspensions. | [21] |
| Implants | Ibuprofen | Chitosan/polycaprolactone | Sustained release for 120 h by diffusion-erosion, 75.3% cell viability. | [22] |
| Hydrogel-based microneedles | Salvia miltiorrhiza | Carboxymethyl chitosan/oxidized pullulan | Simple penetration of HFM-1 into infant porcine skin was demonstrated because of its remarkable mechanical characteristics. Drug release was rapid from HFM-1, hence suitable for transdermal delivery. | [23] |
| Hydrogel | Berberine chloride hydrate | Chitosan/puerarin | Increased rheological properties due to dense structure of CS/PUE18 hydrogels. Dual anti-inflammatory and antimicrobial activities with pH-dependent drug release | [24] |
| Thermogel | Dexamethasone | Hexanoyl glycol chitosan | Versatile release kinetics with no initial burst release. Excellent residual stability without any ear-related side effects and could deliver high concentrations of the drug to the inner ear. | [25] |
| Hydrogel | Acyclovir | Chitosan/β-cyclodextrin/methacrylic acid (MAA) and N′ N′-methylenebis- acrylamide (MBA) | Zero-order kinetics of drug release with pH-dependent swelling behavior. After acute oral toxicity studies, no significant behavioral, histopathological, and clinical changes were observed in Wistar rats. Increased bioavailability as compared to acyclovir suspension at a dose of 20 mg/kg in rabbit plasma. | [26] |
| Hollow capsule | Gemcitabine/curcumin | Chitosan/poly(ethylene glycol dimethacrylate-co-methacrylic acid) | pH-dependent drug delivery at pHs 5.5 and 7.4. Encapsulation efficiency was above 84%, and release efficiency was 82%. Good cytotoxicity towards HCT-116 colorectal cancer cell lines. | [27] |
| Nanohybrid | 5-fluorouracil | Chitosan/collagen/gold nanoparticles/biotin-quat188-chitosan (Bi-QCS-AuNPs@collagen) | Bi-QCS-AuNPs@collagen overcame the low drug load capacity of AuNPs from 64.675 to 87.46% as well as excellent anti-inflammatory activity in macrophage cell lines (RAW264.7). Moreover, in comparison to free 5-FU, the nanohybrid improved drug activity by 3.3-fold in HeLa cell lines and 6.2-fold in A549 cell lines, respectively. | [28] |
| Nanoparticles | Voriconazole | Chitosan | The drug loading in NPs ranged from 75% to 90%. Sustained-release profile in rat skin model and exhibited antifungal activity against C. albicans. | [29] |
| Films | Ciprofloxacin | Chitosan/chitosan-depolymerization products | Low acetylated and molecular weight CDP-based films exhibited reduced swelling and ciprofloxacin released in a controlled manner for up to 54% in 24 h in a pH-dependent manner. | [30] |
CONCLUSION
Chitosan is a remarkable natural biopolymer with a unique combination of properties that make it highly valuable across a range of industries. While native chitosan has limitations, the synthesis of its derivatives has successfully expanded its utility, creating materials with tailored properties for specific applications. From life-saving biomedical devices to environmentally friendly water purification systems, chitosan and its derivatives are playing an increasingly important role in addressing global challenges in health, sustainability, and resource management. Continued innovation in synthesis and functionalization will undoubtedly lead to new and exciting applications, further cementing chitosan’s status as a key biopolymer for the future.
Conflict of Interest
The author declares no conflicts of interest.
Authorʼs Contribution
The author Rajkumari Thagele confirms sole responsibility for the conception and design of the study, data collection, analysis and interpretation of results, and manuscript preparation.
REFERENCES
| [1] | Izadi H, Asadi H, Bemani M. Chitin: a comparison between its main sources. Front Mater. 2025;12:1537067. Doi: 10.3389/fmats.2025.1537067. |
| [2] | Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–632. Doi: 10.1016/j.progpolymsci.2006.06.001. |
| [3] | Aranaz I, Alcántara AR, Civera MC, Arias C, Elorza B, Heras Caballero A, et al. Chitosan: an overview of its properties and applications. Polymers (Basel). 2021;13(19):3256. Doi: 10.3390/polym13193256. |
| [4] | Kumar MNVR. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. Doi: 10.1016/S1381-5148(00)00038-9. |
| [5] | Issahaku I, Tetteh IK, Tetteh AY. Chitosan and chitosan derivatives: recent advancements in production and applications in environmental remediation. Environ Adv. 2023;11:100351. Doi: 10.1016/j.envadv.2023.100351. |
| [6] | Sutharsan J, Boyer CA, Zhao J. Physicochemical properties of chitosan edible films incorporated with different classes of flavonoids. Carbohydr Polym Technol Appl. 2022;4:100232. Doi: 10.1016/j.carpta.2022.100232. |
| [7] | Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers (Basel). 2018;10(3):267. Doi: 10.3390/polym10030267. |
| [8] | Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49(4):780–792. Doi: 10.1016/j.eurpolymj.2012.12.009. |
| [9] | Tan H, Ma R, Lin C, Liu Z, Tang T. Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J Mol Sci. 2013;14(1):1854–1869. Doi: 10.3390/ijms14011854. |
| [10] | Al-Nemrawi NK, Altawabeyeh RM, Darweesh RS. Preparation and characterization of docetaxel-PLGA nanoparticles coated with folic acid–chitosan conjugate for cancer treatment. J Pharm Sci. 2022;111(2):485–494. Doi: 10.1016/j.xphs.2021.10.034. |
| [11] | Manimaran D, Elangovan N, Mani P, Subramanian K, Ali D, Alarifi S, et al. Isolongifolene-loaded chitosan nanoparticles: Synthesis and characterization for cancer treatment. Sci Rep. 2022;12:19250. Doi: 10.1038/s41598-022-23386-4. |
| [12] | Shakeran Z, Keyhanfar M, Varshosaz J, Sutherland DS. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for methotrexate delivery in breast cancer treatment. Mater Sci Eng C. 2021;118:111526. Doi: 10.1016/j.msec.2020.111526. |
| [13] | Mohite P, Puri A, Munde S, Dave R, Khan S, Patil R, et al. Potential of chitosan/gelatin-based nanofibers in delivering drugs for the management of varied complications: a review. Polymers (Basel). 2025;17(4):435. Doi: 10.3390/polym17040435. |
| [14] | Zhu X, Yu Z, Feng L, Deng L, Fang Z, Liu Z, et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates antitumor chemoimmunotherapy in lung cancer. Carbohydr Polym. 2021;268:118237. Doi: 10.1016/j.carbpol.2021.118237. |
| [15] | Wang Y, Yu H, Wang S, Gai C, Cui X, Xu Z, et al. Targeted delivery of quercetin by chitosan-based nanoparticles sensitizing paclitaxel-resistant lung cancer cells. Mater Sci Eng C. 2021;119:111442. Doi: 10.1016/j.msec.2020.111442. |
| [16] | Naruphontjirakul P, Viravaidya-Pasuwat K. Development of anti-HER2-targeted doxorubicin core–shell chitosan nanoparticles for breast cancer treatment. Int J Nanomedicine. 2019;14:4105–4121. Doi: 10.2147/IJN.S198552. |
| [17] | Samadi A, Haseli S, Pourmadadi M, Rashedi H, Yazdian F, Navaei-Nigjeh M. Curcumin-loaded chitosan-agarose-montmorillonite hydrogel nanocomposite for the treatment of breast cancer. In: Proc 27th Natl 5th Int Iranian Conf Biomed Eng (ICBME); 2020 Nov 26–27; Tehran, Iran. Piscataway (NJ): IEEE; 2020. p. 148–153 Doi: 10.1109/ICBME51989.2020.9319425. |
| [18] | Aslzad S, Heydari P, Abdolahinia ED, Amiryaghoubi N, Safary A, Fathi M, et al. Chitosan/gelatin hybrid nanogel containing doxorubicin as an enzyme-responsive drug delivery system for breast cancer treatment. Colloid Polym Sci. 2023;301:273–281. Doi: 10.1007/s00396-023-05066-5. |
| [19] | Wang Y, Qian J, Yang M, Xu W, Wang J, Hou G, et al. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for synergistic chemotherapy of breast cancer. Carbohydr Polym. 2019;225:115206. Doi: 10.1016/j.carbpol.2019.115206. |
| [20] | Zhang M, Zhang F, Li C, An H, Wan T, Zhang P. Application of chitosan and its derivative polymers in clinical medicine and agriculture. Polymers (Basel). 2022;14(5):958. Doi: 10.3390/polym14050958. |
| [21] | Zewail M. Folic acid-decorated chitosan-coated solid lipid nanoparticles for oral treatment of rheumatoid arthritis. Ther Deliv. 2021;12(4):297–310. Doi: 10.4155/tde-2020-0123. |
| [22] | Yang Y, Wu H, Fu Q, Xie X, Song Y, Xu M, et al. 3D-printed poly-caprolactone–chitosan-based drug delivery implants for personalized administration. Mater Des. 2022;214:110394. Doi: 10.1016/j.matdes.2022.110394. |
| [23] | Wei H, Liu S, Chu Y, Tong Z, Yang M, Guo Y, et al. Hydrogel-based microneedles of chitosan derivatives for drug delivery. React Funct Polym. 2022;172:105200. Doi: 10.1016/j.reactfunctpolym.2022.105200. |
| [24] | Yuan H, Li W, Song C, Huang R. Injectable supramolecular nanofiber-reinforced chitosan hydrogel with antibacterial and anti-inflammatory properties. Int J Biol Macromol. 2022;205:563–573. Doi: 10.1016/j.ijbiomac.2022.02.015. |
| [25] | Yu Y, Kim DH, Suh EY, Jeong SH, Kwon HC, Le TP, et al. Injectable glycol chitosan thermogel formulation for inner ear drug delivery. Carbohydr Polym. 2022;278:118969. Doi: 10.1016/j.carbpol.2021.118969. |
| [26] | Malik NS, Ahmad M, Alqahtani MS, Mahmood A, Barkat K, Khan MT, et al. β-cyclodextrin–chitosan-based hydrogels with pH-responsive properties for controlled release of acyclovir. Drug Deliv. 2021;28(1):1093–1108. Doi: 10.1080/10717544.2021.1921074. |
| [27] | Kazemi-Andalib F, Mohammadikish M, Divsalar A, Sahebi U. Hollow microcapsules with pH-sensitive chitosan/polymer shell for curcumin and gemcitabine delivery. Eur Polym J. 2022;162:110887. Doi: 10.1016/j.eurpolymj.2021.110887. |
| [28] | Hongsa N, Thinbanmai T, Luesakul U, Sansanaphongpricha K, Muangsin N. Modified chitosan/collagen-coated gold nanoparticles for 5-fluorouracil delivery. Carbohydr Polym. 2022;277:118858. Doi: 10.1016/j.carbpol.2021.118858. |
| [29] | Shah MKA, Azad AK, Nawaz A, Ullah S, Latif MS, Rahman H, et al. Formulation development, characterization and antifungal evaluation of chitosan nanoparticles for topical delivery of voriconazole. Polymers (Basel). 2022;14(1):135. Doi: 10.3390/polym14010135. |
| [30] | Affes S, Aranaz I, Acosta N, Heras Á, Nasri M, Maalej H. Chitosan derivative-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int J Biol Macromol. 2021;182:730–742. Doi: 10.1016/j.ijbiomac.2021.04.014. |